Research
Metal-sulfur batteries
Metal sulfur batteries are considered next generation batteries with higher theoretical energy densities compared to Li-ion batteries. Also, metal sulfur batteries are sustainable as sulfur is abundant and does not aggravate the already delicate critical mineral supply chain. Despite these advantages, the complex working mechanisms of metal sulfur batteries that entail solid-liquid-solid conversion processes, limit the commercialization of these batteries. The formation of soluble metal polysulfides that migrate between the cathode and anode (known as the shuttling effect), leads to loss of active material, loss of capacity and overall reduced performance are undesirable.
Our group is addressing this challenge by engineering cathode materials to optimally host sulfur to prevent leaching out of polysulfides into electrolyte systems. In this case, we are exploring different architectures of porous carbon materials coupled with appropriate electrocatalysis pathways, that can immobilize these polysulfides. Gel polymer electrolytes (GPEs) are being investigated as a promising alternative to liquid electrolytes in Li(Na)-S batteries due to their potential to address safety concerns and improve battery performance. GPEs offer a balance of ionic conductivity, electrochemical stability, and mechanical properties, making them suitable for various battery systems, including those using sodium metal anodes. In our group, the gel-polymer separators are developed on the base of Polyethyleneoxide (PEO) and Poly(vinylidenfluorid-co-hexafluorpropylen) - PVDF-HFP. Furthermore, we are developing modified, innovative separators to limit the shuttle effect in sodium-sulfur Li(Na)-S batteries at room temperature. The further development of these solutions is crucial for the future commercialization of metal-sulfur batteries.

Na-ion batteries
Among the “post-lithium” systems, the Na-ion battery is the most advanced one and has been recently developed at an industrial scale by several companies and startups around the globe. Despite the substantial similarity of Na with Li, the shift from one technology to the other is not straightforward as the reactivity of the two alkali metals is different: Moving from Li to Na (and K) requires a complete reassessment of materials and components: what works with lithium may not work with sodium and vice versa. However, the energy density of Na-ion batteries is lower than that of Li-ion batteries. Therefore, researchers are trying to improve the energy density by replacing the hard carbon anode with metallic Na anode. But metallic Na is very reactive, raising safety concerns. Another way to improve energy density is to remove the anode, creating an anode-free sodium battery. Despite the energy density, this type of battery allows us to improve safety and reduce production costs.

From a fundamental point of view, we work on developing novel high-capacity and sustainable cathode materials, such as layered oxides or other types of materials (e.g., phosphates) and the formulation of novel electrolytes. We are also studying pre-sodiation with sacrificial additives to tackle the irreversible capacity loss and the sodium deficiencies of Na-based cathode materials.
Zn-batteries
Zinc-ion batteries are emerging as a promising “beyond-lithium” solution for stationary energy storage. As a divalent metal, zinc is abundant, environmentally benign, and delivers a high theoretical capacity.
Because the Zn²⁺/Zn standard potential (−0.76 V vs. SHE) is far less negative than that of Li/Li⁺ (−3.0 V vs. SHE), water-based electrolytes can be employed. These aqueous electrolytes are cheaper, safer, and more sustainable than the organic carbonates used in most lithium-ion cells. Their main limitation is the narrow electrochemical window imposed by water electrolysis, which confines zinc-ion technology largely to stationary applications where lower energy density is acceptable.
Most batteries use a “rocking-chair” configuration in which the same ion shuttles between electrodes. We instead explore hybrid-ion (“accordion-style”) architectures, where different ions are involved for each electrode reaction. This design promises higher operating voltages while retaining the benefits of metal Zinc Anode. Unlocking its full potential requires a detailed understanding of the charge-storage mechanisms. Our group studies these processes with a suite of advanced analytical techniques.
Commercialization is further hampered by the zinc anode’s tendency to form dendrites during cycling, which can cause short circuits and cell failure. We are tackling this challenge through two complementary approaches: incorporating additives into the electrolyte and developing gel-electrolyte systems—both of which show considerable promise in our ongoing research.

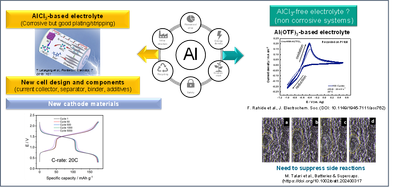
Aluminum-based batteries
Among possible “beyond lithium” candidates, Aluminum is the most abundant one, and, due to its tri-valence, it can theoretically provide three times more charge per redox center as compared to Lithium. However, multivalent systems are much more complicated than monovalent ones due to their higher charge density and the poorer solubility of their salts. The standard electrolyte for Aluminum batteries is based on a combination of AlCl3 with an ionic liquid chloride salt (e.g., Ethyl-Methyl-Imidazolium Chloride, EMImCl). An excess of AlCl3 is required to create the “active species” Al2Cl7-, which enables Aluminum plating and stripping at the negative electrode. However, this formulation is very acidic and corrosive, restricting the use of available current collectors to Molibdenum, Tungstenum, or metal nitrides such as TiN or Cr2N. In addition to this, the electrolyte is highly reactive toward the binder; it dissolves many cathode materials and even destroys the aluminum foil itself upon repeated cycling.
In our group, in collaboration with the Karlsruhe Institute of Technology and the University of Ulm, we aim to push the boundaries of research on Aluminum batteries by addressing several of these issues and tackling them from different fronts: on one side, we work on the formulations of non-corrosive electrolytes, which would have the advantage of overcoming the corrosion problem, but the drawback of having poor performance as compared to standard electrolytes. On the other hand, we are working on optimizing and stabilizing the different components of the electrode and of the cell in a way that they can be “compatible” with the corrosive AlCl3/Ionic Liquid electrolyte.
From electric double-layer capacitors to pseudocapacitors and hybrid configurations
To meet growing demands for electric automotive and regenerative energy storage applications, researchers worldwide have sought to increase the power density of batteries and the energy density of electrochemical capacitors. Hybridizing battery capacitor electrodes can overcome the energy density limitation of conventional electrochemical capacitors because they employ both the system of a battery-like (redox) and a capacitor-like (double-layer) electrode, producing a larger working voltage and capacitance. Our goal is to hybridize materials at a molecular level by incorporating the battery-like material with a carbonaceous capacitor-type material and to adopt different strategies for incorporating faradaic materials:



