Research
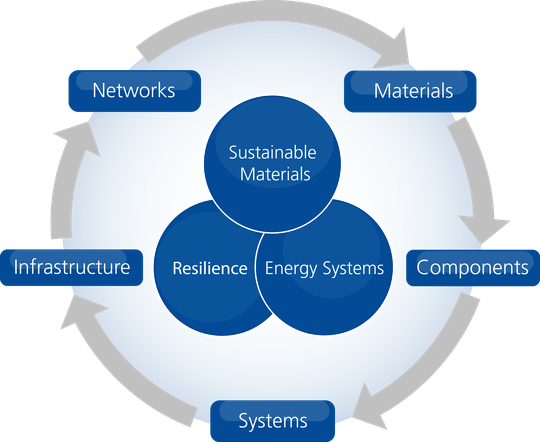
The department emphasizes three key topics for research:
- Sustainable Materials: the research of materials, components, and systems with a focus on energy-efficient usage and resource-efficient production processes.
- Energy Systems: designing solutions to meet society’s demand for energy sources and storage technologies which are available, renewable, and dependable.
- Resilience Engineering: developing engineering technologies for critical infrastructure to enhance resistance against short-term detrimental effects and increase adaptive capabilities to better cope with long-term evolutionary changes. Such technologies address challenges associated with e.g. natural disasters or climate change.
Figure 1 illustrates how INATECH’s key topics of research act in concert to create a value-added chain. While Sustainable Materials focusses on materials and components research, Energy Systems and Resilience Engineering address issues related to entire systems, infrastructure, or networks of infrastructures..